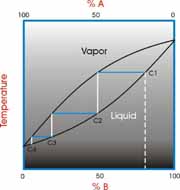
What is an Azeotrope?
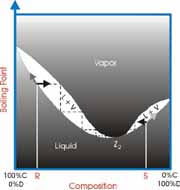
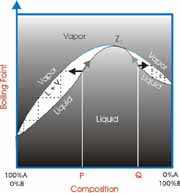
Ideally, when a mixture of two liquids is distilled the lower boiling material vaporizes first and is collected separately from the second material. However, sometimes the two materials form a constant boiling mixture and are collected together even though they have different boiling points. This is exactly what the Greek word azeotrope means, “constant boiling”.
When it comes to solvent recycling, this can prevent the collection of a pure solvent.
The most common example is the azeotrope between water and ethanol (grain alcohol). Water boils at 100 ºC and ethanol boils at 78.3 ºC. The mixture will boil at 78.2 ºC and have a composition of 95% ethanol and 5% water by volume. This is a binary azeotrope because it involves two components. It is also a minimum boiling azeotrope because the boiling point of the mixture is below either of the two pure components.
Another common binary azeotrope found in solvent recycling is acetonitrile and water. This is a common mixture used for HPLC analysis. Acetonitrile boils at 81.6 ºC and water boils at 100 ºC. The mixture forms an azeotrope which boils at 76.1 ºC and which is composed of 86% acetonitrile and 14% water.
There are also azeotropes between three components and these are called ternary azeotropes. A common ternary azeotrope found in solvent recycling is Acetonitrile, water and methanol. This is a common mixture used in HPLC analysis. Acetonitrile boils at 81.6 ºC, water boils at 100 ºC and methanol boils at 64.5 ºC. When mixed together the three form an azeotrope that boils at 65-70 ºC and is composed of 44% acetonitrile, 52% methanol and 4% water.
How can we know if a mixture will form an azeotrope? There are two general guidelines. First, the closer in boiling points the components are the more likely they will form an azeotrope. Second, the greater the difference in polarity the components are the more likely that they will form an azeotrope. However, these general rules are only guidelines. Reference books containing lists of known azeotropes are available to check for azeotropes between components of your waste mixture.
One easy way to prevent azeotropes is to segregate waste solvents. By not mixing different solvents together, the possible formation of many azeotropes is avoided. In fact good waste segregation is the foundation of any good solvent recycling program.
| Component A | Component B | Boiling Point A | Boiling Point B | Azeotrope Boiling Point | Azeotrope Wt.% A |
| Water | Methanol | 100 °C | 64.5 °C | Non-Azeotrope | |
| Water | Ethanol | 100 °C | 78.3 °C | 78.2 °C | 4 % |
| Water | Isopropanol | 100 °C | 82.3 °C | 80.3 °C | 12.6 % |
| Water | Acetone | 100 °C | 56.2 °C | Non-Azeotrope | |
| Water | Acetonitrile | 100 °C | 81.6 °C | 76.1 °C | 16% |
| Water | Ethyl Acetate | 100 °C | 77.2 °C | 70.3 °C | 8.5 % |
| Water | Acetic Acid | 100 °C | 118 °C | Non-Azeotrope | |
| Water | THF (tetrahydrofuran) | 100 °C | 66 °C | 65 °C | 5% |
| Water | Dichloromethane | 100 °C | 40 °C | 38.1 °C | 1.5% |
| Component A | Component B | Boiling Point A | Boiling Point B | Azeotrope Boiling Point | Azeotrope Wt.% A |
| Dichloromethane | Methanol | 40 °C | 64.5 °C | 37.8 °C | 92.7 % |
| Dichloromethane | Hexane | 40 °C | 68.8 °C | Non-Azeotrope | |
| Dichloromethane | Ethyl Ether | 40 °C | 34.6 °C | 40.8 °C | 70 % |
| Dichloromethane | Acetone | 40 °C | 56.2 °C | Non-Azeotrope | |
| Acetone | Hexane | 56.2 °C | 68.8 °C | 49.8 °C | 59 % |
